Blog
Management representative audit checklist – ISO 9001 / ISO TS 16949
- Post author: orbit
- Post published: July 11, 2014
- Post category: Checklist for Management representative / Checklist for Management representative data / Checklist for Management representative for environment / Checklist for Management representative for food safety / Checklist for Management representative for health and safety / Checklist for Management representative for ISO 22001 audit / Checklist for Management representative for safety / Checklist for Management representative form / Checklist for Management representative format / Checklist for Management representative iso 13485 / Checklist for Management representative iso 22001 / Checklist for Management representative records / Checklist for Management representative sample / Checklist for Management representative template / EXCEL format / Formats / ISO / TS 16949 / ISO 13485 / ISO 14001 / iso 14001 Management representative checklist / ISO 22001 / ISO 9001-2015 / ISO 9001:2008 / JPEG format / Management representative checklist / Management representative checklist example / Management representative checklist form / Management representative checklist format / Management representative checklist sample / Management representative checklist sample form / Management representative checklist template / OHSAS 18001 / PDF formats / QUALITY SYSTEM / Word Document Format
A management representative audit checklist is a document to make sure the entire internal management system is complying the standards requirements. It may help to provide objective evidence of the implementing and effectiveness of the system. The checklist may include the planning, scheduling, and executing internal quality management audits points.
Management representative audit checklist
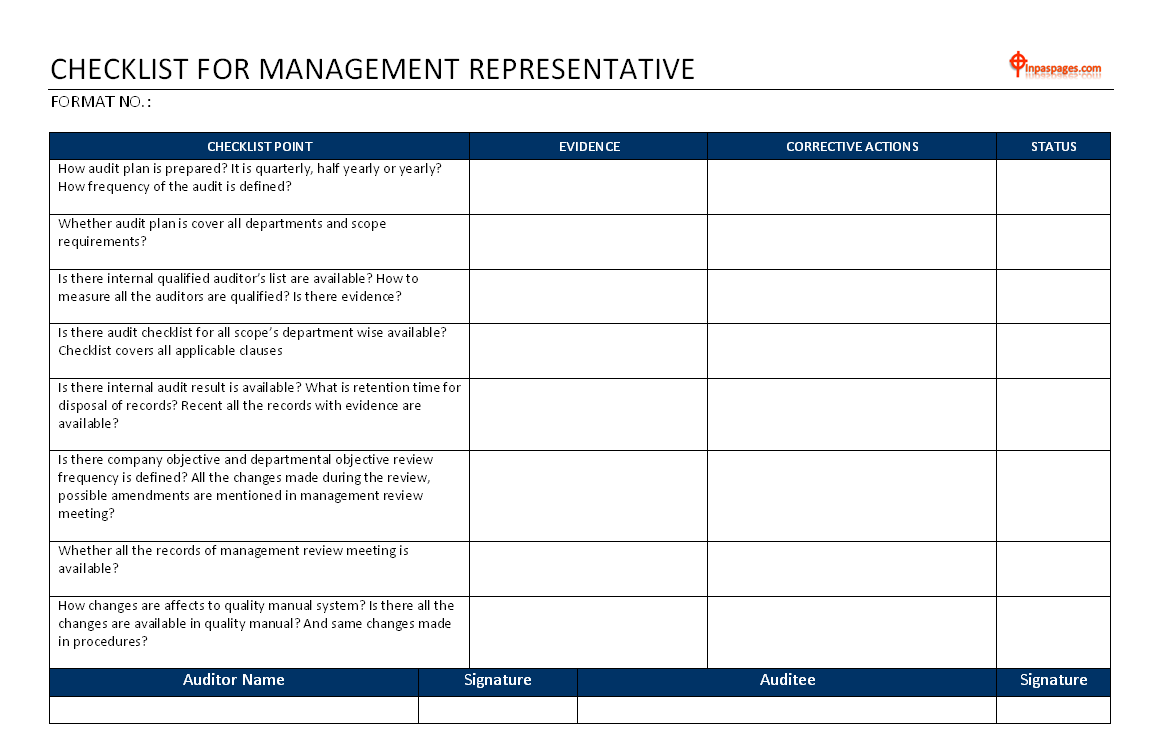
The checklist may includes following:
- How audit plan is prepare? It is quarterly, half yearly or yearly? How frequency of the audit is define?
- Whether audit plan is cover all departments and scope requirements?
- Is there internal qualify auditor’s list are available? How to measure all the auditors are qualify? Is there evidence?
- Is there audit checklist for all scope’s department wise available? Checklist covers all applicable clauses
- Is there internal audit result is available? What is retention time for disposal of records? Recent all the records with evidence are available?
- Is there company objective and departmental objective review frequency is define? All the changes made during the review, possible amendments are mention in management review meeting?
- Whether all the records of management review meeting is available?
- How changes are affects to quality manual system? Is there all the changes are available in quality manual? And same changes made in procedures?
What would MR do to complete all of the checklist?
An annual Audit plan shall be prepare by the MR to include internal audits for QMS.
The audit plan will show the:
- Scope of the audit (departments to be audit)
- Plan month of the audit,
- Status of the audit (i.e. schedule, complete, report issue, close out, etc.).
Audits shall be perform by auditor(s) chosen by the Management Representative on the basis of:
- Master list of Auditors
- Independence from responsibility for the area being audit.
Preparatory planning for audits is the responsibility of the chosen auditor(s).
- Review of relevant procedures, previous audit findings and Non-Compliance Reports,
- Preparation of an audit checklist to assist with audit investigations, (Product Audit & Process Audit is compulsory)
Internal audits shall be conduct with the primary intention of measuring the Company’s compliance with:
- 6-month business plans / objective / targets
- Quality system procedures, work instructions, policy, etc,
- requirements of the quality/ environmental system standard
- applicable Statutory or Regulatory requirements,
- Product or process quality standards
The Auditee must document (on the audit report or attach form) and take action to correct Non-Compliance found during an audit. The appropriate section of the Audit or Non-Compliance Report shall be sign off by the Auditee upon completion of the plan action and then forward to the Management Representative.
The MR or Auditor will decide if any further action require, including whether the audit Schedule needs to be revise including a follow-up audit. Details shall be recorded in the appropriate section of the audit report. If all Non-Compliance related to an internal audit have been closed out, the Management Representative shall close out the audit by marking the Audit Schedule accordingly.
EXAMPLES, SAMPLES & FORMATS: Download
Related:
- QUALITY MANAGEMENT SYSTEM PROCESSES
- BENEFITS OF QUALITY MANAGEMENT SYSTEM – ISO 9001:2015
- QUALITY CONTROL PLAN
- MANAGEMENT AUDIT SHEET
- APPOINTMENT OF MANAGEMENT REPRESENTATIVE FOR IMS
- APPOINTMENT LETTER TO MANAGEMENT REPRESENTATIVE FORMAT
- EHS AUDIT GENERAL QUESTIONS / CHECKING POINTS TO MANAGEMENT REPRESENTATIVE
- PROCESS SAFETY MANAGEMENT CHECKLIST
- REPRESENTATIVE APPOINTMENT FORM
orbit
Orbit is a expert in solutions of industrial problems viz quality, production, planning, inspection etc. Orbit is writes about manufacturing's problems and its state solutions for quality lovers..


